Environmental Biology Group
The Environmental Biology Group develops and deploys capabilities to sample, detect, and respond to biological threats in the environment. Our diverse group includes expertise in the areas of environmental sampling, molecular biology, microbiology, ecology, chemistry, quality assurance, and population genetics. We strive to improve the time-to-detect, efficiency, sensitivity, and specificity of analytical tools used for counterterrorism (ie. BioWatch), food safety, and response/recovery efforts. Our current research projects involve developing rapid viability methods for select agents, fielding a mobile biological laboratory for special event monitoring, and developing methods for detecting biothreats in complex environmental samples.
Research Areas
- Biosurveillance through Environmental Monitoring
- Biothreat Response Vehicle: A Field Deployable Laboratory
- COVID-19 Testing
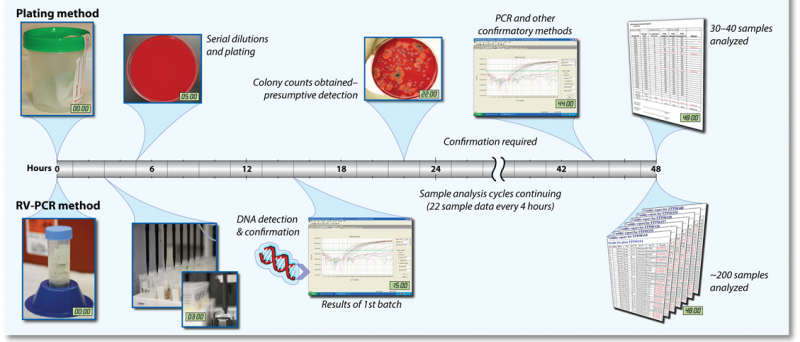
- Rapid Viability—Polymerase Chain Reaction (RV-PCR)